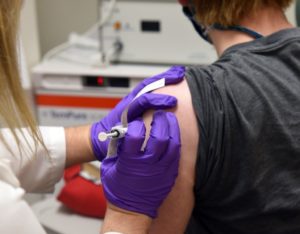
Novavax, a vaccine development company headquartered in Gaithersburg, says its COVID-19 vaccine candidate showed 89.3% efficacy in UK clinical trials. The UK study enrolled more than 15,000 volunteers, according to a statement from Novavax on Thursday. More details will be released as data comes in, the company said. Phase 2b results in South Africa showed […]
emergency use authorization
State Officials Explain What’s in Pfizer’s COVID-19 Vaccine
What’s in Pfizer’s COVID-19 vaccine? Dr. David Marcozzi, COVID-19 Incident Commander for the University of Maryland Medical System, explained the answer to Marylanders at a press conference with Gov. Larry Hogan on Tuesday. Pfizer’s vaccine was granted emergency use authorization (EUA) by the FDA last Friday. Marcozzi said there are four components that make it […]


Engage us on Facebook
Follow us on Twitter
Tweets by @mymcmedia